This week in PLOS Biology
In PLOS Biology this week, you can read about access to research data, regulation of cell asymmetry and septum formation in Caulobacter, and unfolding and refolding RNA.
Access to Data – The Publishers’ Role
A new community perspective piece by Jennifer Lin and Carly Strasser calls on publishers to promote and contribute to increasing access to data. This call to action emerged from a summit attended by data experts from diverse stakeholder groups; the Perspective outlines eight recommendations and a set of suggested action items for publishers to enhance access to data.
***Two-for-One Offer on Caulobacter Papers!!!
1. Stopping Cell Division in Case of Damage
Cells have evolved sophisticated mechanisms for repairing their DNA and maintaining genome integrity. A critical aspect of this process is the arrest of cell cycle progression until the genome has been repaired. In new research, Joshua Modell, Michael Laub and colleagues explore the mechanisms behind inhibition of cell division in the bacterium Caulobacter crescentus. They report a new mechanism, in addition to the previously known ‘SOS response,’ which involves the inhibition of division septum formation by DidA. Read more in the accompanying Synopsis.
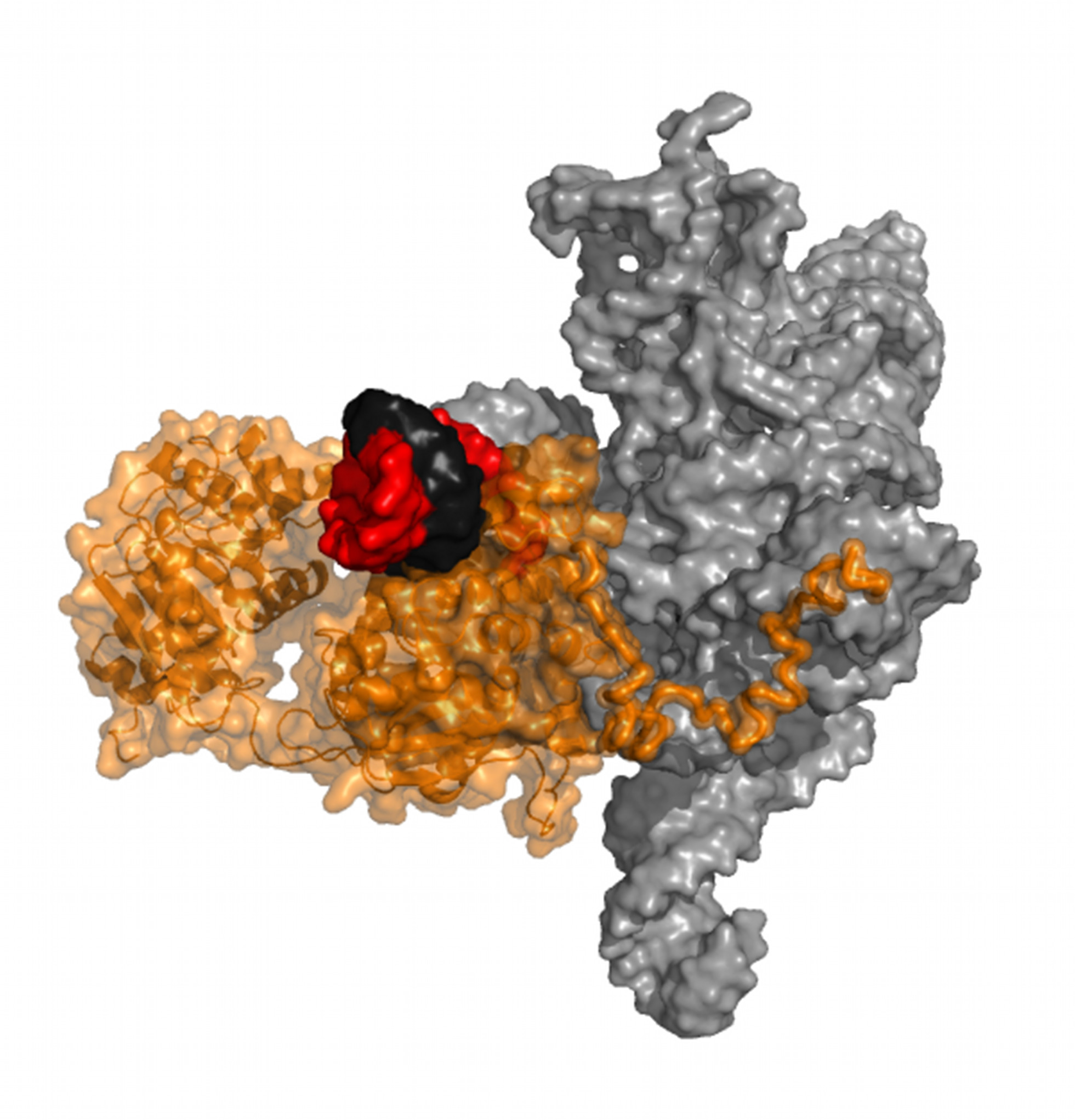
2. Reversal of Fortune: Flipping a Kinase
A fundamental strategy of asymmetric cell division is to place different signalling molecules in each nascent daughter cell, which then go on to drive the two cells toward different fates. In Caulobacter crescentus, a bacterial model used to study this process, a critical molecular driver is a “response regulator” protein called DivK, which interacts with pseudo-histidine kinase called DivL. Normally, information flows from histidine kinases, which transfer a phosphate signal to their response regulator; however in this issue of PLOS Biology, Seth Childers, Qingping Xu, Lucy Shapiro, and colleagues show that DivL has taken an unusual evolutionary twist that allows it to serve as a highly specific sensor for the phosphorylation state of DivK, thereby reversing the normal flow of information. Read more in the accompanying Synopsis.
Unfolding and Refolding RNA
RNAs function in many essential cellular processes, often requiring them to form a specific three-dimensional structure. However they also have a propensity to become trapped in non-functional, misfolded structures. DEAD-box proteins are known to help by disrupting such misfolded structures. In a new research article, Cynthia Pan, Rick Russell and colleagues reveal how DEAD-box proteins capture transiently exposed RNA helices, preventing any tertiary contacts from reforming and potentially destabilizing the global RNA architecture. Helix unwinding by the DEAD-box protein then allows the product RNA strands to form new contacts. Read more in the accompanying Synopsis.